Researchers have developed a powerful computational tool, CausalXtract, that can uncover the complex cause-and-effect relationships between cells in biological systems. This innovative technology has the potential to revolutionize our understanding of cell-cell interactions and pave the way for advancements in various fields, from cancer research to drug development.
Unraveling Cellular Dynamics
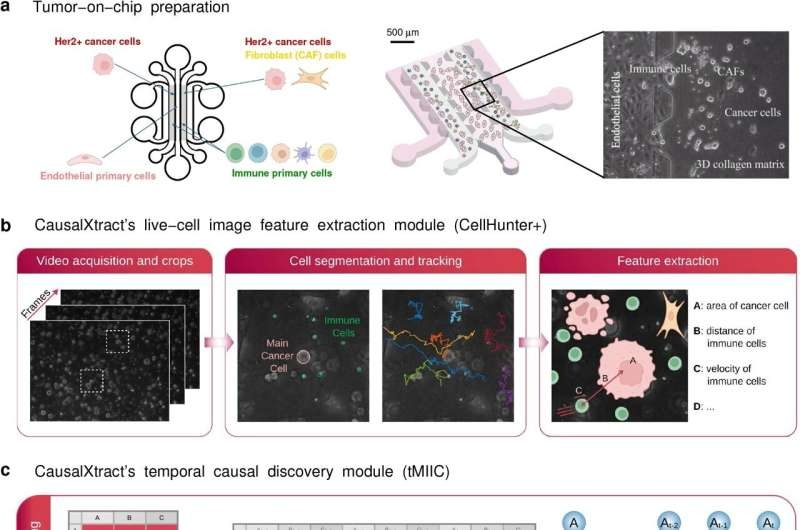
Live cell imaging has become a powerful tool for revealing novel information concerning the morphology, health and interactions of cells under diverse experimental conditions. Yet few tools have existed to precisely map such cellular features in a cause-and-effect manner—until now.
To tackle the above challenge, we have introduced CausalXtract tool that construct time-unfolded causal networks with multiple nodes for each variable and different durations. This methodology adds the importance of the links between consecutive time steps in the data to allow for a more profound understanding of underlying causal processes.
Through its benchmarking with artificial datasets and real biological data (from a tumor-on-a-chip model), the researchers have shown it to be efficient compared to existing methods, running multiple orders of magnitude faster. This breakthrough sets the stage for a future where we can further understand the complex communication that occurs in biological systems.
Deciphering Life-Relevant Reflections
In order to truly test how well CausalXtract can parse real-world data, the research team used time-lapse imaging data from a tumor-on-a-chip model equally mimicking tumor architecture and microenvironment on chip scale. The model provided them with a wide range of cellular features, including geometry, velocity, cell division and death rates, and short- and long-term cell-cell interactions.
The reconstruction of the time-unfolded causal network from this data yielded a number of interesting discoveries. The model first substantiated what was already known—that trastuzumab prompts cancer cells to die and then facilitates interactions between a tumor and immune cells. But it also revealed that CAFs protect cancer cells from death in response to a drug, findings that explain—at least in part—how CAFs may help to blunt treatment efficacy.
This corresponded to the opposing effects at different time points that CausalXtract was also able to identify. It was able to show, for example, that a cell’s eccentricity and its distance from the nearest vertex (how much it deviates from being perfectly circular and how close it is to an “edge”) are statistically different at different points in the cell division process; The late stages of the cell division process are associated with an increase in cellular eccentricity, but this is preceded by a decrease in eccentricity 2–4 h before the onset of cytokinesis (i.e. after cells have committed to divide). This showcases the capacity of the tool to reveal new and time-lagged causative links between cellular properties.
Conclusion
In consequence, CausalXtract constitutes an important advance of the state-of-the-art in computational biology. The versatility of this tool could open new ways to analyze live-cell imaging data cost-effectively, providing fundamental knowledge in the wide field from cancer biology to immunotherapy screens. The amount of live-cell imaging data is growing; therefore, interpretation methods like CausalXtract gaining flexibility and high power will be important to extract the full benefit of this information-rich data.
