Scientists at Sanford Burnham Prebys have made a major breakthrough in understanding how the crucial cellular machinery responsible for recycling most proteins in our cells, known as proteasomes, are assembled. This discovery could pave the way for new treatments targeting various cancers and age-related diseases.
The Vital Role of Proteasomes
Without proteasomes, our cells would spiral out of control. They account for degrading and recycling about 80% of the proteins in our cells, making them mini protein grinders.
Who wants to ensure that the novelty has only old, damaged or misaligned proteins, which out source might have been eliminated. Changes to this balance can lead to cancers or age-related disease.
This makes the assembly of proteasomes inside our cells a highly sophisticated process, which means that discovering how this takes place could inform entirely new therapeutic strategies to disrupt these essential structures.
Dissecting the Molecular Choreography of Proteasome Formation
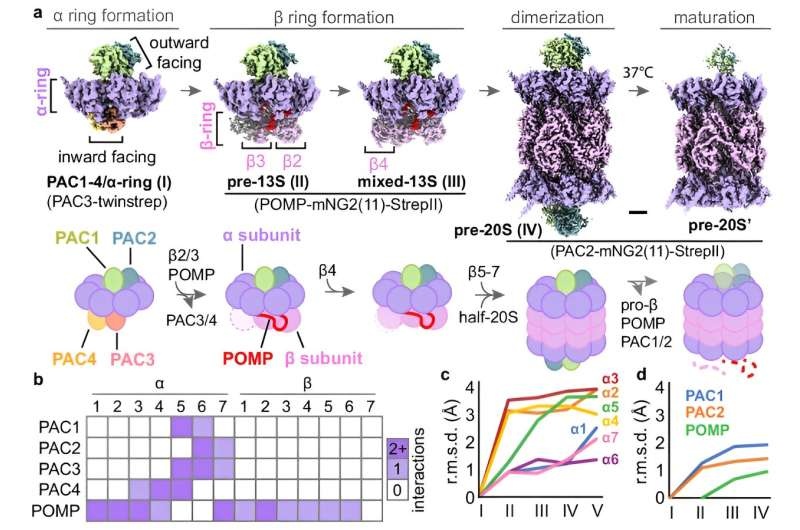
By employing the latest gene editing methodologies and today’s best-in-class cryogenic electron microscopy (cryo-EM), study lead Dr. Jianhua Zhao and graduate student Hanxiao Zhang offer a new glimpse into how one of the most abundant forms found in human, animals, and plant cells—the 20S proteasome—comes together.
A tense, involved one-on-one molecular dance of many steps, with the help of a variety of assistant proteins — called chaperones — works to guide the intricate shape change leading to formation of the 20S proteasome’s barrel-like structure.
While previous studies has already made spectrograms in the middle of this process, the Sanford Burnham Prebys team mapped out those steps more definitively and also revealed initial and endpoint parts have never seen before to show step by step how these crucial cellular substrates are constructed.
The science team created 3D pictures showing how the chaperone proteins interact with the 20S core; these three components are alpha subunits and beta subunits. They could also ‘see’ the entire biogenesis of the proteasome in a step by step process – from how an alpha subunit ring forms, to coupling the four protein rings into a barrel-like structure.
Conclusion
Research conducted by the group at Sanford Burnham Prebys has been instrumental in providing a window into the assembly process of proteasomes, among the most vital player controlling recycling and disposal of nearly half-million proteins in our cells.
The discovery could also have implications for cancer and age-related diseases that may one day be controlled or could help scientists design and engineer entire proteasomes in labs to make them work better. The researchers argue that the work could be a starting point for broad-based applications in mapping host-pathogen interactions, which speaks to just how significant this finding is.
