Scientists from Rice University have discovered a breakthrough that could change the way in which scientists think about cholesterol’s role in our cell membranes and cellular receptors. The work, soon to be published in the Journal of Physical Chemistry, may lead to greater insight into diseases that are tied to membrane organization.
New Light on the “Dark Matter” of Cholesterol
Cholesterol´s structure within biomembranes, which are dynamic and complex structures composed of lipids (including cholesterol) and proteins, was for long difficult to be understood by researchers. Until now, the methods used to produce these unusual particles have resisted full characterization, but a fresh study led by Jason Hafner, of Rice University’s departments of physics and astronomy and chemistry appears to have solved the riddle.
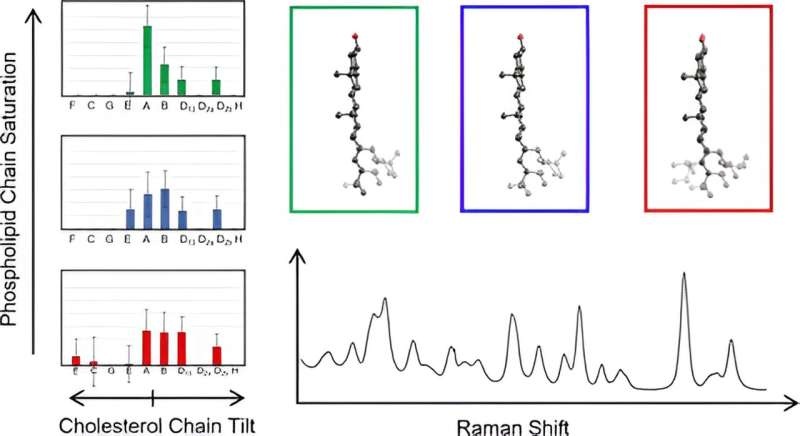
Raman spectroscopy, a powerful analytical tool that scatters laser light off molecules and provides detailed vibrational spectra to measure the vibrations of each individual cholesterol molecule has enabled researchers to observe the unique vibrations of each cholesterol in their body and provide new information about their structure. The team found that when the spectra for 60 distinct, computed cholesterol structures this parameter clustered these molecules due to how each eight-carbon chain bends towards or away from the plane of the rings.
The discovery is the first time researchers have been able to directly measure cholesterol chain structures in a natural membrane context. All the cholesterol molecules in each group showed remarkably similar low-frequency spectra, which “is the surprise we saw,” Hafner said. That allowed us to perform a coarse-grained analysis and fit experimental data of membrane cholesterol chains.
Connection to Disease Pathogenesis
The ramifications of this finding are enormous, as this could provide a new avenue to begin teasing out how cholesterol affects the membranes of cells and their receptors. One outcome of this is that researchers will have a deeper understanding of potential targets for the variety of diseases associated with membrane organization, such as cancer.
Hafner adds, “Cell membranes are key to the immune response while also playing a major role in diseases with poorly understood etiologies such as cancer. The positioning of cholesterol in the membrane is essential for a well-organized structure but also has an influence on, e.g., the receptor movement and distribution within a lipid bilayer — knowing all these details would help us to understand how diseases work.
One example of these is cancer cells, which are observed to have different membrane organization that may result in the functionality of their receptors and signaling pathways. This work, which deconstructs the intricate kinship between cholesterol and membrane organization, unveils avenues for a novel approach to treating cancer and other diseases linked to modifications in membrane maturation.
Conclusion
The discoveries made by the Rice University team have deep implications for understanding cholesterol’s role in cell membranes and ultimately human health. The observation of the cholesterol in its natural state presented here assigns a new horizon for future investigations, mainly in diseases attributed to membrane organization such as cancer. While the scientific community builds on these discoveries, we might just be approaching a paradigm shift in how to address some of the most difficult health crises we currently face.
