Researchers from the RIKEN Center for Biosystems Dynamics Research have made a significant discovery in understanding the structural secrets of antiepileptic drugs, which could lead to the development of even more effective treatments for this debilitating neurological disorder.
Unraveling the Mysteries of SV2A
A new study shows that the answer might be what target these drugs are acting on, as a protein called synaptic vesicle glycoprotein 2A (SV2A) has been identified in research and is shown to be crucial for this kind of response. Levetiracetam and brivaracetam both bind to SV2A selectively, marking their role in neuronal functioning, including inhibiting electrical impulses within the brain.
SV2A is anchored in synaptic vesicles, which are little pouches primed to release the chemicals neurons use to communicate across gaps (or synapses) between nerve endings. The exact role played by SV2A is not well defined, but it is thought to be involved in synaptic transmission how signals are sent from one neuron to another.
Curiously, SV2A is also a receptor for the deadly botulinum neurotoxin, deemed to be one of the most toxic substances in existence. This serendipitous connection has given researchers a new tool they can use in their efforts to unmask the anti-epileptic drug’s structural secrets.
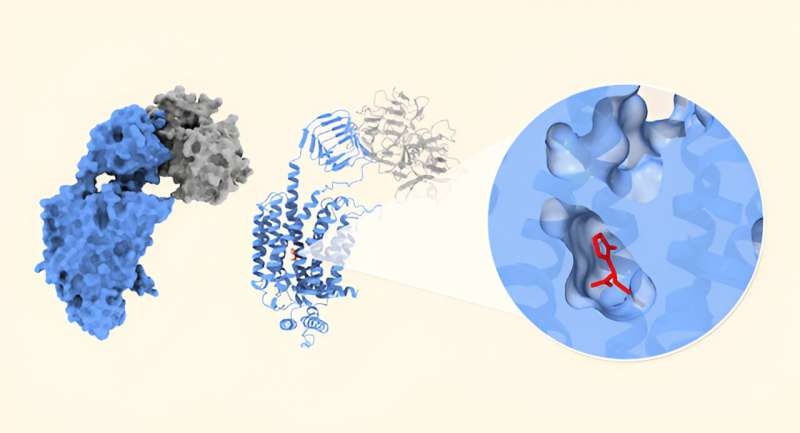
Cryo-Electron Microscopy Reveals Structural Insights
These researchers used cryo-electron microscopy to solve the structures of levetiracetam and brivaracetam bound with SV2A, and they were able to image these complexes in the presence or absence of the botulinum neurotoxin.
These findings represent a major advance in how SV2A is working. These data support the hypothesis that SV2A functions as a membrane transporter and demonstrate that levetiracetam and brivaracetam can suppress this activity. This knowledge could be important for understanding the functions of SV2A during epileptogenesis, particularly in cases associated with genetic mutations of SV2A.
The discovery of how to take advantage of the botulinum neurotoxin receptor-binding domain as a marker for cryo-EM image analysis completely transformed the game in this field, as it allowed the researchers to align SV2A particle images more accurately and work out the high-resolution cryo-EM structure of the protein.
Conclusion
These structural insights will help design more efficient antiepileptic drugs if our results are reproduced in clinical cases. The breakthrough discovery allows scientists to begin designing new treatments that better target this important protein and will allow the millions of people affected by epilepsy to have new options for treating their disease.
