Exciting new research reveals the true nature of helical peptide sequences and their potential to represent a bold new horizon for creating restorative biomaterials and drugs.
Twisting peptides
Proteins are the building blocks of life, which makes their intricate structure important for function. In proteins, this is especially common and important for helices.
The helical structures are formed by the arrangement of the amino acids—known as the building blocks of proteins—that make up coilin. The twist and orientation of these helices can affect the properties and behavior of the protein to a large extent, making them very interesting entities in terms of research.
A team of researchers has now discovered another type of chiral information that controls the deployment of these helical structures in peptides, the shorter pieces of proteins. The realization of this basic principle will give scientists a more nuanced understanding of the intricate dance steps that proteins choreograph and new tools to engineer novel biomaterials and therapeutics.
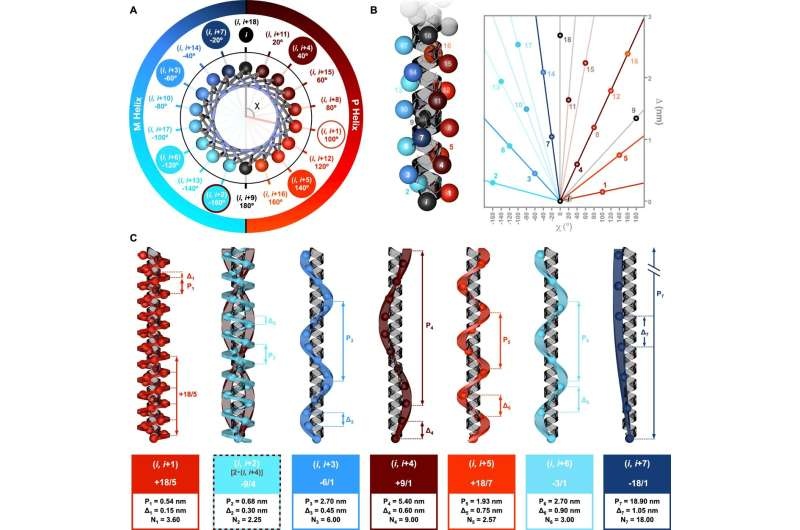
Memories of the Symmetry in Helices
The research, published in the prestigious journal Nature Communications, was led by Dr. Julián Bergueiro and his team at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS) The researchers used a combination of computational methods and state-of-the-art spectroscopic techniques to probe the complex dependence of various kinds of helical structures on one another.
A symmetry model uncovered in the study is part of what explains how these helixes form and work. Analysis of the differences in charge and length of the arrays of amino acids classified particular exo-helical topologies, all of which matched theoretical predictions perfectly [89].
While the insight into how the sequence of amino acids can affect the properties of peptide helices is valuable, it remains important to have a deeper understanding of this. This could allow them to program the arrangement of these building blocks when designing specific helical structures with desired properties, which might find applications in fields ranging from medicine and biotechnology to materials science.
Conclusion
In a landmark publication in Nature Communications, this study has opened new dimensions to our understanding about how amino acid sequences dictate the helical formations of peptides. The discovery of a hidden layer of chiral information for the underlying helical structures promises to inspire new biomaterials and therapeutics using the molecular building blocks. The research represents a landmark in the field of peptide chemistry and opens up new possibilities for molecular engineering, smart Houston.—Ends…
