Researchers have made a groundbreaking discovery in understanding how bacteria strengthen their cell walls to protect themselves from environmental dangers, opening new avenues for developing novel antibacterial therapies.
Uncovering the Protective Mechanism
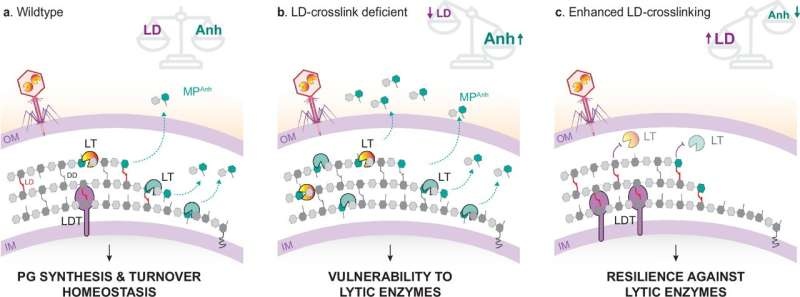
Scientists at Umea University, together with researchers at Cornell University in the US have found that many bacteria divide as soon as move but also there is a special chemical compound to it. Published in Nature Communications, the study shows that LD-crosslinked peptidoglycan lodged against lytic transglycolases — enzymes that chew up cell wall – prevents these proteins from carrying out their work.
The finding is significant because these enzymes are important in the bacterial growth process and defense system. Bacteria can use them for shedding cell wall fragments which modulate the host immune system and kill competing bacteria. The bacteria can use the enzymes to control their activity and thus protect themselves, from the immune system as well as other attacks of competing bacteria or against viruses.
The Delicate Balance of Cell Wall Maintenance
Bacteria are sheltered by their peptidoglycan cell wall, which allows them to endure the internal turgor pressure and external aggression from natural killers such as other bacteria and viruses. Bacteria need a mix of these activities to construct and maintain their cell wall. One such family of enzymes is lytic transglycolases capable of breaking the peptidoglycan chains.
Just how such enzymes are regulated, however, has remained a mystery. A new study by the Umeå University research group of Felipe Cava, in collaboration with researchers at Cornell University published in Nature Microbiology sheds crucial light. The findings this is not only a new mode to gain insight into the intricate interactions of peptidoglycan modifying enzymes but also that LD-crosslinking in the cell wall inhibitors activity of lytic transglycosylase gives molecular perspective on the complexity of bacterial cell wall and may provide potential options for novel antibacterial therapies.
Conclusion
Understanding how bacteria protect themselves from such threats, including the immune system and attacks from other microorganisms, is essential for addressing food safety. The understanding gleaned from the study may help pave the way for new therapies that interfere with the crosslinking process used by LD a strategy that would likely undermine one of the bacteria’s lines of defense and make them more susceptible to antibiotics, as well as our immune system. The discovery points to the crucial need for research in the basic features of bacterial life as this could lead to new ways of preventing and treating infections.
