Hypersensitivity pneumonitis (HP) is an immune-mediated lung disease that can progress to pulmonary fibrosis, a serious condition characterized by the activation of lung fibroblasts. Researchers have now uncovered crucial differences in the gene expression and splicing patterns of these fibroblasts in HP patients compared to healthy individuals. Their findings shed light on the unique molecular characteristics that may contribute to the development and progression of this complex lung disorder. This research highlights the importance of understanding the cellular mechanisms underlying HP and could pave the way for the identification of novel therapeutic targets. Hypersensitivity pneumonitis, Pulmonary fibrosis, Fibroblasts
Uncovering Molecular Differences in Hypersensitivity Pneumonitis Fibroblasts
Hypersensitivity pneumonitis (HP) is a challenging lung disease caused by an exaggerated immune response to various environmental triggers. In some cases, HP can progress to pulmonary fibrosis, a serious and often fatal condition characterized by the overactive proliferation and activation of lung fibroblasts. Understanding the unique features of these fibroblasts in HP could provide crucial insights into the disease’s pathogenesis and potential therapeutic targets.
Researchers from the National Institute of Respiratory Diseases in Mexico City set out to investigate the expression of 16 genes involved in the immune response and the splicing patterns of their corresponding proteins in lung fibroblasts derived from HP patients and healthy controls.
Altered Gene Expression Profiles in HP Fibroblasts
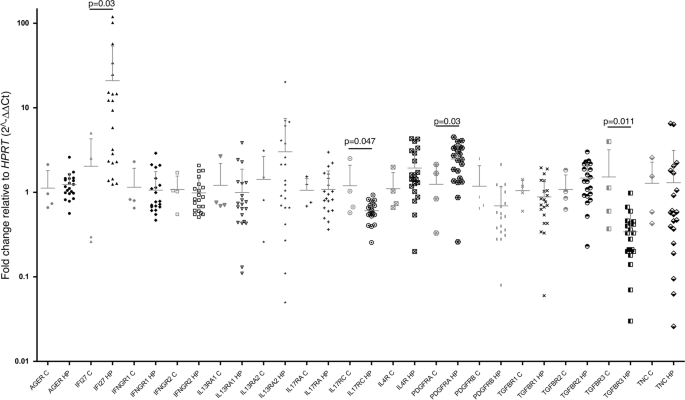
The analysis revealed some striking differences in the gene expression patterns of HP fibroblasts compared to the control group. HP fibroblasts showed significantly increased levels of the IFI27 and PDGFRA genes, while the expression of IL17RC and TGFBR3 was decreased.
IFI27 is a protein associated with the immune response and cellular processes like proliferation and cell death. The researchers found that IFI27 was highly expressed not only in the HP fibroblasts but also in the fibrotic areas of the lungs, suggesting a potential profibrotic role for this molecule.
Splicing Variants Reveal Altered AGER Expression
In addition to changes in gene expression, the researchers also analyzed the splicing patterns of the selected genes. Interestingly, they found that the AGER gene, which encodes the receptor for advanced glycation end products (RAGE), showed an imbalance between the full-length and soluble isoforms in HP fibroblasts. The soluble variant, which lacks the transmembrane domain, was increased compared to the full-length form.
RAGE is an important molecule in the lung, and its altered expression has been linked to various pulmonary diseases, including idiopathic pulmonary fibrosis. The shift towards the soluble isoform in HP fibroblasts may have implications for the disease’s progression and warrants further investigation.
Potential Profibrotic Role of IFI27
To delve deeper into the functional significance of the elevated IFI27 expression, the researchers overexpressed this gene in normal lung fibroblasts. They found that IFI27 overexpression led to a decrease in cell number over time, increased levels of pro-caspase 3 (a marker of apoptosis), and enhanced expression of α-smooth muscle actin (a hallmark of myofibroblast differentiation).
These findings suggest that IFI27 may play a profibrotic role in lung fibroblasts, potentially by promoting some of the characteristics associated with the myofibroblast phenotype, a key driver of pulmonary fibrosis.
Implications and Future Directions
The insights gained from this study provide a more comprehensive understanding of the unique molecular features of lung fibroblasts in HP. The altered expression of genes like IFI27, PDGFRA, and the imbalance in AGER isoforms highlight the complex interplay between the immune response and fibroblast biology in this disease.
These findings could pave the way for the identification of novel biomarkers and therapeutic targets for HP. Further research is needed to elucidate the precise mechanisms by which these molecular changes influence the fibroblast phenotype and contribute to the development and progression of pulmonary fibrosis in HP.
Author credit: This article is based on research by Ana Lilia Torres-Machorro, Carina Becerril, Everardo Hernández-Plata, Erika Rubí Luis-García, Mariel Maldonado, Iliana Herrera, Miguel Negreros, Fernando Hernández-Sánchez, Criselda Mendoza-Milla, Miguel Gaxiola, Remedios Ramírez, Annie Pardo, Ivette Buendía-Roldán, Moisés Selman, José Cisneros.
For More Related Articles Click Here
