In a remarkable feat, researchers have uncovered a novel protective mechanism in the cell walls of bacteria, shedding light on their remarkable resilience and potential implications for developing new treatments.
Bacteria’s Hidden Fortress
Here are those outstanding organisms, bacteria already known that they can adapt and make themselves at home in almost all sorts of environments. At its core is the unassuming though formidable peptidoglycan cell surface. This elaborate structure not only helps bacteria withstand pressures and adversities from their environment but also has important functions in bacterial growth and division.
The Peptidoglycan chain is assembled and disassembled by enzymes on either side of the cell wall. A specific group of enzymes, the lytic transglycolases, cleave these chains. Yet, the underlying mechanisms controlling these enzymes sing their praises and remain a mystery till currently.
Unveiling the Secret of LD-Crosslinking
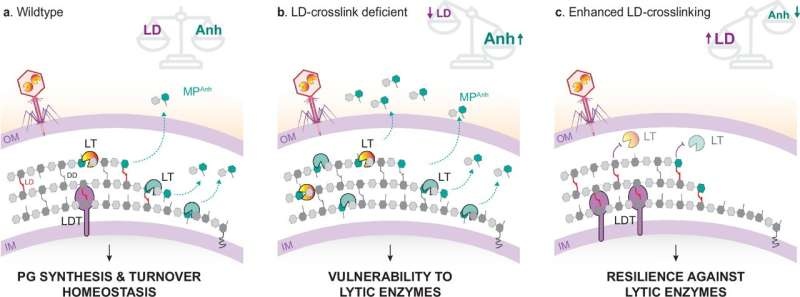
The new bacterium protective chambers that have been discovered by a research team from Umeå University in Sweden and Cornell University in the United States constitute previously unknown bacterial cell walls mechanisms, as detailed in their scientific study. As Estaquier and colleagues conclude in a paper published online this week in Nature Communications, a particular type of crosslinking in the cell wall—LD-crosslinking—may underlie the long-known activity of lytic transglycolases.
Moreover, this has grave consequences. This leads to an elegant relationship for the bacteria, as some release cell wall fragments that modulate the host immune system by using these enzymes while others synthesize them to kill off competing bacteria. This enzyme activity makes it possible for the bacteria to avoid the immune system and fight off other microorganisms more efficiently.
“Until now, there was an important missing piece in the role of LD-crosslinking in cell wall homeostasis,” says the principal investigator and Umeå University researcher Felipe Cava. “By a simple structural change in their cell wall, we have demonstrated that bacteria can enhance significantly its overall robustness against this and other environmental stresses,” said Isaiah Arkin from the Department of Life Sciences at BGU.
Conclusion
Unraveling this process reveals not only a novel aspect of bacterial cell wall homeostasis but also potential targets for new antibacterial therapies. Striking the LD-crosslinking vulnerability could impair bacterial defense, increasing susceptibility to antibiotics and immune clearance. This could open the door to new possibilities in our war against antibiotic-resistant superbugs, those dreaded greatest threats to life and health in the developed world.
