Researchers have developed a groundbreaking live-cell imaging system that provides unprecedented insights into how natural killer (NK) cells eliminate target cells, such as cancer cells or transplanted organ cells. By combining 2D static and 3D microfluidic experiments, the scientists were able to capture the complex interactions between NK cells and endothelial cells, which line the blood vessels. This cutting-edge technology offers a more sensitive and comprehensive view of NK cell functions compared to conventional cytotoxicity assays. The findings could lead to improved cancer immunotherapies and better management of transplant rejection. Natural killer cells play a vital role in the body’s immune response, while endothelial cells form the inner lining of blood vessels.
Revolutionizing Cell Interaction Imaging
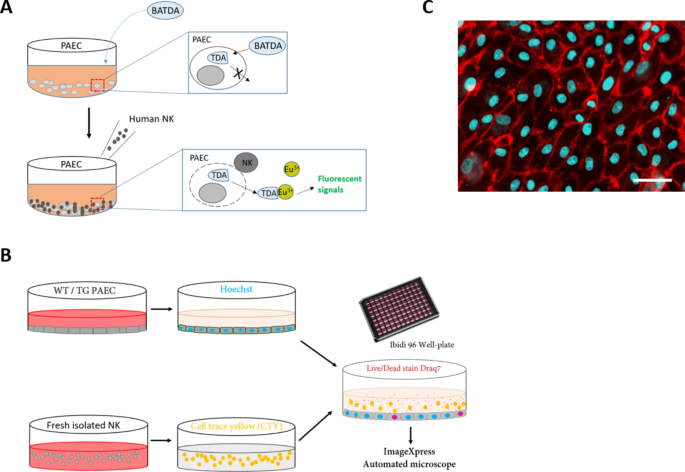
Researchers from the University of Geneva have developed a novel live-cell imaging system that allows them to study the complex dance between natural killer (NK) cells and endothelial cells in unprecedented detail. NK cells are a type of immune cell that play a crucial role in fighting viral infections, cancers, and transplant rejection. Endothelial cells line the inner walls of blood vessels and are one of the first cell types to interact with NK cells in the body.
Uncovering the Subtleties of NK Cell Killing
The researchers used their new imaging system to compare the behavior of NK cells when interacting with normal endothelial cells versus those that had been activated by the inflammatory molecule tumor necrosis factor (TNF). They found that NK cells displayed more dynamic and aggressive behavior when encountering the TNF-activated endothelial cells, exhibiting increased adhesion, migration, and cytotoxicity.
Interestingly, the researchers also discovered that the majority of endothelial cell death induced by NK cells occurred through a process called apoptosis, where the target cells undergo programmed cell death. This is in contrast to necrosis, which involves the uncontrolled breaking apart of the cell. Understanding the precise mechanisms of NK cell killing is crucial for developing more effective cancer immunotherapies and managing transplant rejection.
Combining 2D and 3D Approaches
The researchers utilized two different experimental setups to study NK cell-endothelial cell interactions. In the 2D static system, they were able to track the movements and killing patterns of individual NK cells in great detail. This revealed that NK cells exhibited distinct migratory behaviors, including small, confined movements, as well as larger, more directed movements.
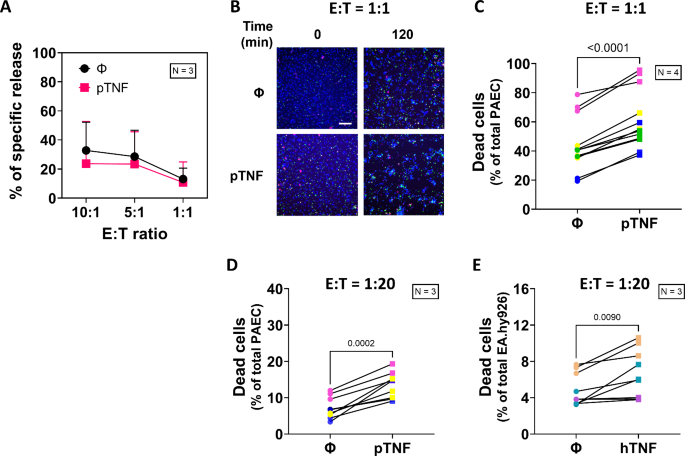
Fig. 2
To better mimic the conditions found in the human body, the researchers also developed a 3D microfluidic system that simulated the flow and shear stress encountered in blood vessels. In this 3D system, the NK cells displayed a range of migratory behaviors, with some cells adhering firmly to the endothelial layer while others moved in various directions, even against the flow.
Implications for Improving Treatments
The researchers believe that their live-cell imaging approach, combining 2D and 3D systems, provides a powerful tool for studying the complex interplay between immune cells and their targets. This knowledge could lead to the development of more effective cancer immunotherapies, as well as better strategies for managing transplant rejection.
By understanding the precise mechanisms by which NK cells eliminate their targets, researchers can potentially find ways to enhance or modulate these processes to improve patient outcomes. Additionally, the 3D microfluidic system could be a valuable tool for testing new drugs or therapies that target the interactions between NK cells and endothelial cells in a more physiologically relevant environment.
A Versatile Platform for Diverse Applications
The researchers emphasize that their live-cell imaging system is not limited to studying NK cells and endothelial cells. The platform can be adapted to investigate the interactions between a wide range of immune cells, such as T cells, B cells, and macrophages, with different target cell types. This versatility makes it a powerful tool for advancing our understanding of the complex dynamics of the immune system and its interactions with various diseases and medical interventions.
Author credit: This article is based on research by Thao Tran, Viktoriia Galdina, Oscar Urquidi, Daniela Reis Galvão, Robert Rieben, Takuji B. M. Adachi, Gisella L. Puga Yung, Jörg D. Seebach.
For More Related Articles Click Here
