A recent study has uncovered a concerning issue with benzoyl peroxide (BPO) acne and rosacea treatments – they can degrade into the known carcinogen, benzene, when stored at room temperature or exposed to UV light. This discovery highlights the critical importance of independent testing and proper storage to protect consumers from harmful contaminants. Benzoyl peroxide is a widely used topical treatment, but the findings suggest a need for reformulation or refrigeration to mitigate the risk of benzene exposure.
Instable Acne Treatments: An Alarming Find
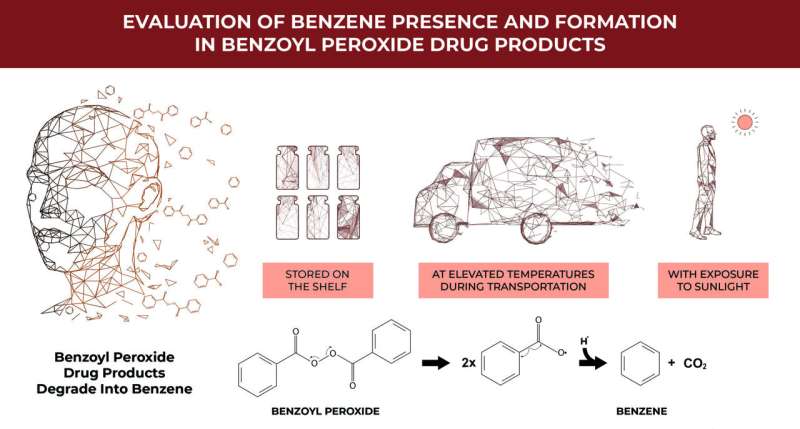
Approximately forty of the leading topical benzoyl peroxide (BPO) acne and rosacea treatments on the market are found to be covered with an unstable layer containing unacceptably high concentrations of benzene, a human carcinogen, according to investigators. This breakdown seems to happen in the products when they sit on a shelf at room temperature, are exposed to higher temperatures, and also when hit with ultraviolet (UV) light that is comparable to sunlight.
According to a study published last month in the Journal of Investigative Dermatology, benzene generation can occur not only during heated storage and transportation, but also from exposure to outdoor levels of sunlight. This is of particular concern as these BPO-containing products are often used by consumers multiple times during long periods, thereby potentially increasing their exposure to this toxic chemical. The lead researcher, David Light, warns that the discovery of benzene formation in acne products containing benzoyl peroxide is a potential health threat.
Refrigeration: A Viable and Low Exposure Solution
However, the researchers discovered that even conventional methods for stabilizing drugs, such as encapsulation, does not prevent benzene formation in BPO drug products. This will require more sustained technical R&D to solve.
Meanwhile, the co-investigator was Christopher G. Bunick, MD, Ph. D., suggests refrigeration as a practical strategy to limit the needless benzene exposure. “Refrigeration may be a practical means of reducing benzene exposure until formulations are introduced to impede the formation of benzene. Furthermore, dermatologists must remind patients to exercise proper use of BPO, with careful education on the risks when exposed to UV rays.
But What Does It Mean for Patients and Doctors?
These observations have serious implications for both patients and healthcare professionals. The use of BPO in combination products for acne and rosacea to some extent could be putting consumers at an unknowable risk from a known human carcinogen because these products can degrade under typical environmental scenarios.
These studies, however, do underscore the importance of extra vigilance for healthcare providers. Dermatologists should counsel patients to take proper precautions when storing and using these products, of course reminding them about the dangers of UV exposure. Lead author from the study, Richard L. Gallo, MD, Ph. D., suggests that “additional research is needed to determine if detection of this potential poison in benzoyl peroxide-containing drug products results in any increased cancer risk.
