Researchers have uncovered a fascinating discovery about a vital enzyme in plants called UBIQUITIN-SPECIFIC PROTEASE 12 (UBP12). This enzyme plays a crucial role in regulating the circadian clock, jasmonate signaling, and epigenetic control of gene expression in Arabidopsis, a model plant species. The researchers identified a novel mutation in UBP12 that changes a single amino acid – a serine residue at position 327. Surprisingly, this mutation enhances the activity of the enzyme, even though disrupting the enzyme’s function typically leads to similar effects.
The study reveals that this highly conserved serine residue across different organisms acts as a regulatory switch, controlling the enzyme’s activity. When this serine is phosphorylated, it inhibits the enzyme’s ability to remove ubiquitin tags from its target proteins. However, the mutation identified in this research prevents this phosphorylation, keeping the enzyme in a constantly active state. This finding provides valuable insights into how post-translational modifications can fine-tune the function of key regulatory enzymes in plants and potentially other organisms.
Unraveling the Secrets of a Critical Enzyme
The circadian clock is the internal timekeeping mechanism that allows plants, and many other organisms, to synchronize their biological processes with the daily cycle of light and dark. This clock is driven by a complex network of gene expression and protein interactions. One of the key players in this network is the enzyme UBIQUITIN-SPECIFIC PROTEASE 12 (UBP12), which plays a crucial role in regulating the stability and abundance of various clock-related proteins.
UBP12 is a deubiquitinating enzyme, meaning it removes ubiquitin tags from target proteins. This modification can affect the stability, localization, and function of these proteins, ultimately impacting the circadian clock and other important plant processes.
Discovering a Hypermorphic Mutation in UBP12
In this study, the researchers used a forward genetic approach to identify a novel mutation in the UBP12 gene in Arabidopsis thaliana, a widely used model plant species. The mutation, designated ubp12-3, resulted in a single amino acid substitution – a serine residue at position 327 was replaced by a phenylalanine (S327F).

Surprisingly, the ubp12-3 mutation caused a short-period circadian phenotype, similar to that observed in the ubp12-1 null mutant, where the entire UBP12 gene was disrupted. This suggested that the S327F mutation was not simply inactivating the enzyme, but rather altering its function in a more complex way.
Uncovering the Dual Roles of UBP12
To better understand the effects of the ubp12-3 mutation, the researchers analyzed the expression of genes that are known targets of UBP12 in different signaling pathways. They found that the ubp12-3 mutant showed the opposite effects compared to the ubp12-1 null mutant, indicating that the S327F mutation was actually enhancing the enzyme’s activity, rather than impairing it.
This was particularly evident in the regulation of jasmonate signaling and epigenetic control of gene expression, where the ubp12-3 mutant displayed a “hypermorphic” phenotype, suggesting increased UBP12 function.
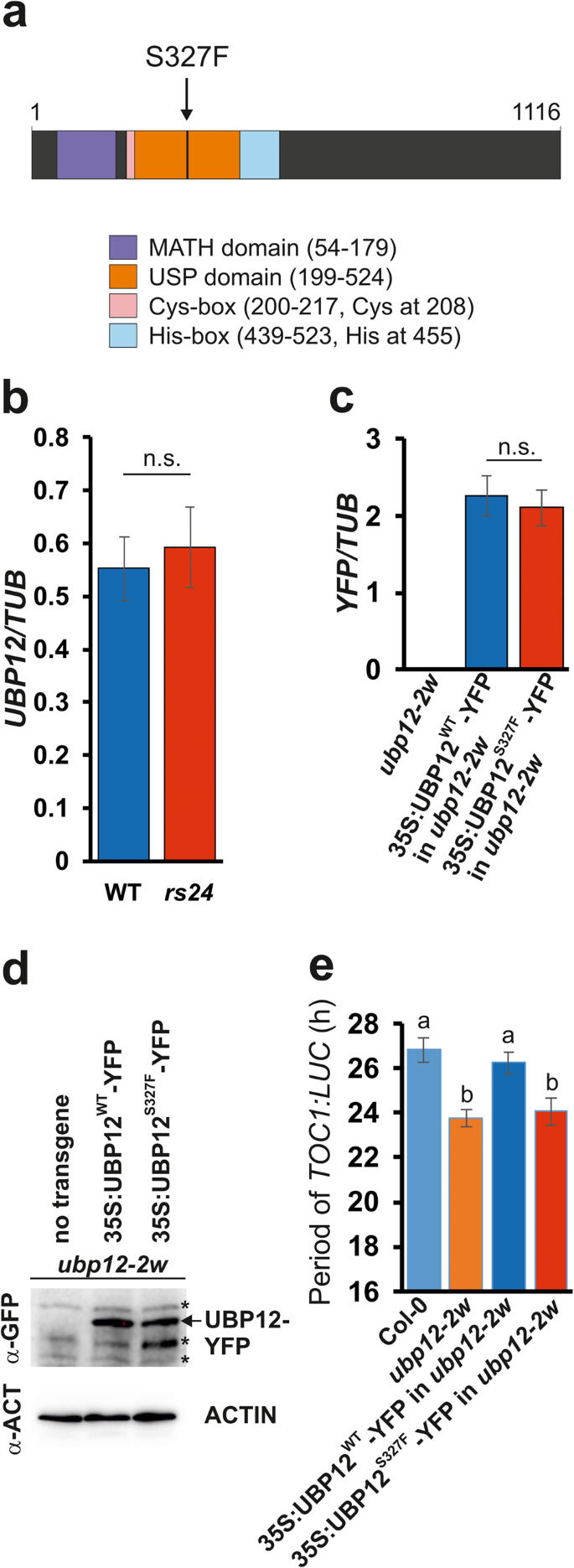
Fig. 2
However, when it came to the circadian clock, both the ubp12-1 and ubp12-3 mutants showed similar short-period phenotypes, indicating that the clock-related functions of UBP12 were affected in a different way.
The Conserved Serine Residue as a Regulatory Switch
The researchers discovered that the serine residue at position 327 in UBP12 is highly conserved across various organisms, from plants to humans. This suggested that this specific amino acid might play a critical role in regulating the enzyme’s activity.
Further investigation revealed that phosphorylation of this serine residue could inhibit the enzymatic activity of UBP12. The S327F mutation in the ubp12-3 mutant prevented this phosphorylation, effectively keeping the enzyme in a constantly active state.

Fig. 3
This finding provides valuable insights into how post-translational modifications, such as phosphorylation, can fine-tune the function of key regulatory enzymes like UBP12. By understanding these mechanisms, researchers can gain deeper insights into the complex regulatory networks that govern important biological processes in plants and potentially other organisms.
Implications and Future Research
The discovery of the critical serine residue in UBP12 has significant implications for our understanding of circadian clock regulation, hormone signaling, and epigenetic control of gene expression in plants. By elucidating the molecular mechanisms underlying the dual roles of UBP12, this research opens up new avenues for investigating the intricate interplay between post-translational modifications and enzyme function.

Fig. 4
Future research in this area could explore the specific kinases and phosphatases responsible for the phosphorylation and dephosphorylation of the conserved serine residue, as well as the structural changes that occur within the enzyme upon modification. Additionally, investigating the impact of this regulatory mechanism on the stability and interactions of UBP12‘s target proteins could provide further insights into the broader implications for plant physiology and development.
Overall, this study highlights the importance of understanding the fine-tuned regulation of key enzymes in complex biological systems. By uncovering the critical role of a single amino acid in modulating the activity of UBP12, the researchers have made a significant contribution to the field of plant biology and laid the groundwork for future discoveries.
Meta description: Researchers uncover a critical serine residue that regulates the activity of a key enzyme, UBIQUITIN-SPECIFIC PROTEASE 12, in plants, providing insights into the fine-tuned control of important biological processes.
For More Related Articles Click Here
